Broadening the Use of Quantitative MRI, a New Approach to Diagnostics
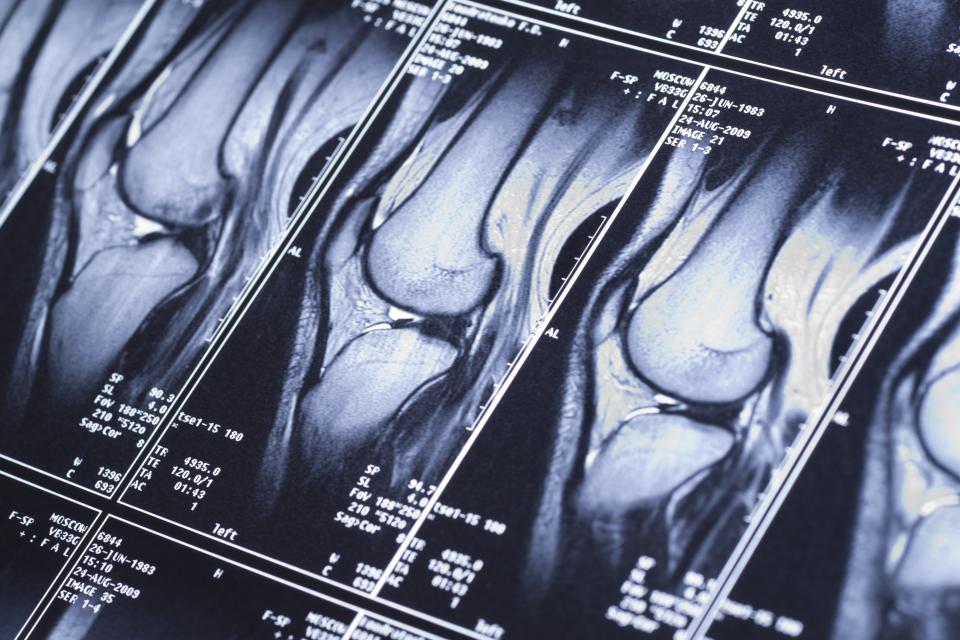
While a traditional MRI captures abnormalities visually, qMRI could highlight valuable non-visual metrics of the chemical structure and makeup of tissues, revealing insights into disease. | Kondor83/iStock
Magnetic resonance imaging has revolutionized medical diagnostics. With MRI — essentially a photograph capturing detail in the contrast between light, dark, and the grays in between — skilled doctors can spot abnormalities ranging from neurologic disorders to ligament damage to cancers.
Recently, a new variation of MRI has emerged: Quantitative MRI (qMRI) captures valuable non-visual metrics of the chemical structure and makeup of tissues, revealing yet greater and increasingly sensitive insights into disease and damage than ever before. Despite its promising advantages, however, qMRI remains an emerging field that has yet to reach widespread use in clinical settings. In this regard, AI could help bring the technology to wider use by more efficiently acquiring qMRI data and bringing greater consistency to how radiologists interpret the images they see.
“In both consistency and speed, artificial intelligence could help bring quantitative MRI to the clinics, but we currently lack the robust data against which to train our AI models. We have no baseline against which to judge our success,” said Akshay Chaudhari, an assistant professor of radiology at Stanford University.
Read the paper, SKM-TEA: A Dataset for Accelerated MRI Reconstruction with Dense Image Labels for Quantitative Clinical Evaluation
Chaudhari, a faculty member of the Stanford Institute for Human-Centered AI (HAI), is principal investigator of a new effort to address that dearth of data. It comes in the form of the Stanford Knee MRI with Multi-Task Evaluation (SKM-TEA), a 1.6 terabyte dataset of more than 25,000 highly annotated knee cross-sections from 155 real-world clinical patients. The hope is that this richer dataset will be widely adopted in the field and used to train new automated AI methods to improve qMRI biomarker estimates and bring the technology into greater clinical use.
Annotation Challenge
In technical terms, qMRI can measure certain biochemical parameters of tissue composition related to the relative water content and extracellular matrix of soft tissues, such as cartilage. “This is quite exciting from a diagnostic and prognostic perspective,” said Arjun Desai, a doctoral candidate in electrical engineering at Stanford and first author of the study. “The relaxation parameters have been shown to be quite promising biomarkers of knee health following acute injuries and for degenerative disorders.”
If, for instance, the scans show increased water content than normal — indicating perhaps a breakdown of the extracellular matrix — it might mean the tissue shows signs of early osteoarthritis. Such mechanisms for diagnosing early osteoarthritis may allow for new promising interventions or lifestyle changes, since there is currently no treatment or cure for the disease.
The SKM-TEA dataset is open source and freely available for download by anyone interested in using it to train AI models. All the images are fully reconstructed and labeled by tissue type (cartilage, meniscus, etc.). Each scan also includes detailed annotation of diagnostic findings of radiological abnormalities and degeneration (ligament tears, cartilage lesions, meniscus damage) for enabling AI-based detection models. Unlike that of previous datasets, the multi-task construction of SKM-TEA affords researchers the flexibility to home in on specific tasks of interest while also benchmark their algorithms in the end-to-end medical imaging workflow.
Identifying and quantifying disease and degeneration demand intensive professional interpretation that is burdensome to gather and maintain and susceptible to variation in interpretation depending on the medical professional doing the labeling. This has slowed access to and analysis of MRI scans, sometimes leading to missed diagnoses, while increasing health care costs.
“All this labeling, even for one relatively simple area of the body such as the knee, is an expensive and time-consuming process that requires knowledgeable humans to manually review and label the data, which can also vary quite a bit depending on who is doing the labeling. In creating SKM-TEA, we provide all that labeling data with an important degree of consistency,” said Desai.
Benchmark Lacking
AI has shown great promise in reconstructing and interpreting MRI images, but before an AI model can improve upon an existing technology, it must first be trained. In effect, the algorithm reads each image and makes predictions about tissue types, damage, and disease it sees and then compares its predictions against known results in the form with fully reconstructed and labeled scans.
Given enough training, AI eventually learns to spot patterns and features in the tissues that even highly trained professionals sometimes cannot. In that ability to learn to spot tissue types and abnormalities, AI can speed and add insight that could make qMRI a mainstay in clinics across the country.
However, because existing benchmarking data for qMRI has been absent, inconsistent, or incomplete, AI researchers hoping to improve the technology had nothing against which to compare their predictions. SKM-TEA fills that void, at least for the knee. To the authors’ knowledge, it is the first dataset to leverage the quantitative nature of qMRI for end-to-end evaluation of the knee. The authors hope to add more labeled scan data to SKM-TEA to make it a dynamic dataset and eventually translate their understandings to other tissue and systems in the body.
“SKM-TEA bridges the gap between image reconstruction and image analysis with thorough, consistent qMRI labeling that will help us — and others — evaluate automated AI methods that could make qMRI both faster and more insightful,” Desai stressed. “That could help bring a powerful new technology to the clinic.”
Stanford HAI's mission is to advance AI research, education, policy and practice to improve the human condition. Learn more.